Vision and Mission

About NovinGene
NovinGene is a biotechnology company operating as the manufacturer of infectious, genetic and onco-genetic qPCR kits. Applying cutting edge technologies, we make sure that our products meet the highest standards for qPCR assays.
a biotechnology company operating as the manufacturer of infectious, genetic and onco-genetic qPCR kits. Applying cutting edge technologies, we make sure that our products meet the highest standards for qPCR assays.

Vision and Mission
NovinGene is dedicated to patient care through innovative solutions for challenging diagnostic problems. At NovinGene we offer high quality and robust kits at competitive prices and strong technical support for each and every molecular laboratory.
NovinGene Story
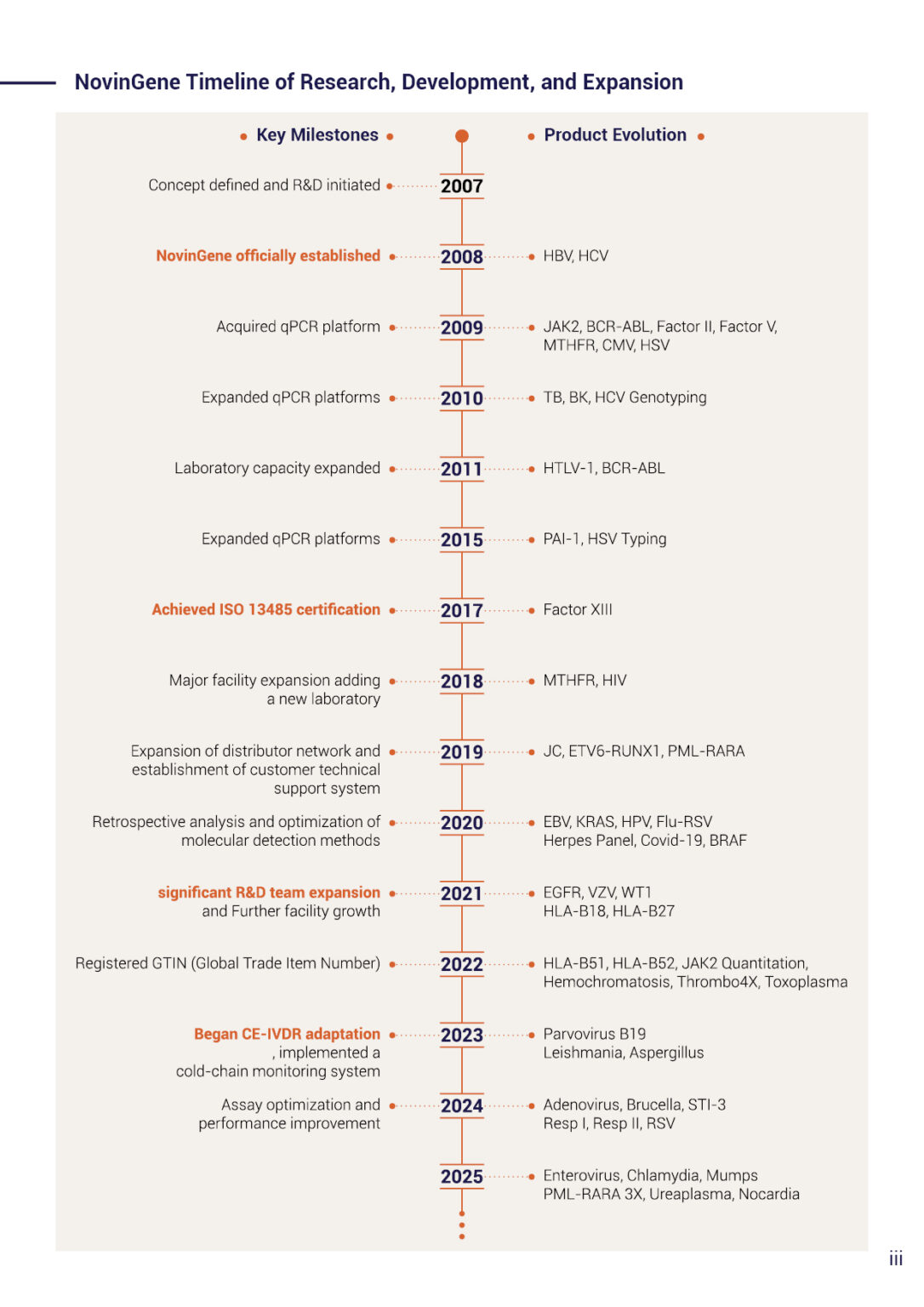
With the help of a professional team of biotech experts, NovinGene company was founded in 2008 as a manufacturer of molecular diagnostic kits. Since then, we have been constantly engaged in the research and development of new products to expand NovinGene portfolio.
Certification
NovinGene Quality Management System received the certificate of ISO 13485:2016, which is special for the design, development and production of qPCR molecular kits.